



Mastitis is the #1 reason dairy cows are treated with antibiotics
Changing the narrative with proactive surveillance using advanced diagnosticsWhile the global livestock industry is trying to reduce the use of antibiotics, it’s time for a fresh look at mastitis and how to keep it out of your herd. One option to consider is more advanced diagnostics, like qPCR testing, which is offering producers an opportunity to be proactive with their herds—to directly monitor the specific mastitis pathogen loads on their dairy farms so they treat only known mastitis infections with the right antibiotic.
Cost of mastitis
Mastitis is estimated to cost the global dairy industry $19.7–$32 billion annually, and the cost of subclinical mastitis in the US alone is expected to exceed $1 billion annually [1]. While the economic impact varies by country, a mastitis case often costs $250 to $500 per clinical case and $100 per subclinical case [2]. Clinical and subclinical cases often result in reduced milk yield and quality, decreased lactation potential, and early culling of lactating cows.

Prudent use of antibiotics can reduce duration of treatment, labor, cost of antibiotics, and the likelihood of pathogen resistance, which can help minimize the economic impact of mastitis on your dairy.
Gram-positive and gram-negative mastitis and mycoplasmas
The basic difference in categories of mastitis pathogens is their physiology or cell structure. Gram-positive mastitis bacteria have a thick cell wall but don’t have a protective outer cell membrane. The cell wall’s membrane is highly selective about how and what components move in and out. Gram-negative mastitis bacteria have a thin cell wall with two membranes that are somewhat selective, and the outer membrane protects the cell wall. Penicillin antibiotics must attack the cell wall to be effective. As a result, gram-positive bacteria tend to be more susceptible to penicillin antibiotics, and gram-negative bacteria are more resistant.
Mycoplasmas do not have a cell wall. They only have a cell membrane, making them much more resistant to antibiotics because there’s no cell wall to attack.
Selective dry cow therapy
Many dairy farms around the world are shifting to selective dry cow therapy (SDCT) to reduce the use of broad-spectrum antibiotics by targeting only cows with known intramammary infections. Previously, at dry-off cows were treated every quarter with antibiotics, regardless of whether or not the animals were infected with mastitis or their somatic cell count (SCC) score.
In 2022, the European Union (EU) banned the use of blanket dry cow therapy (some EU countries banned it earlier) and turned to SDCT. The EU’s goal is to reduce antibiotic therapy use. A 2021 systemic review and meta-analysis showed SDCT can reduce antibiotics at dry-off by 66% [3]. However, from a producer’s perspective, the success of dry-off is important to help ensure healthy cows and increased milk production during a cow’s second and third lactations.
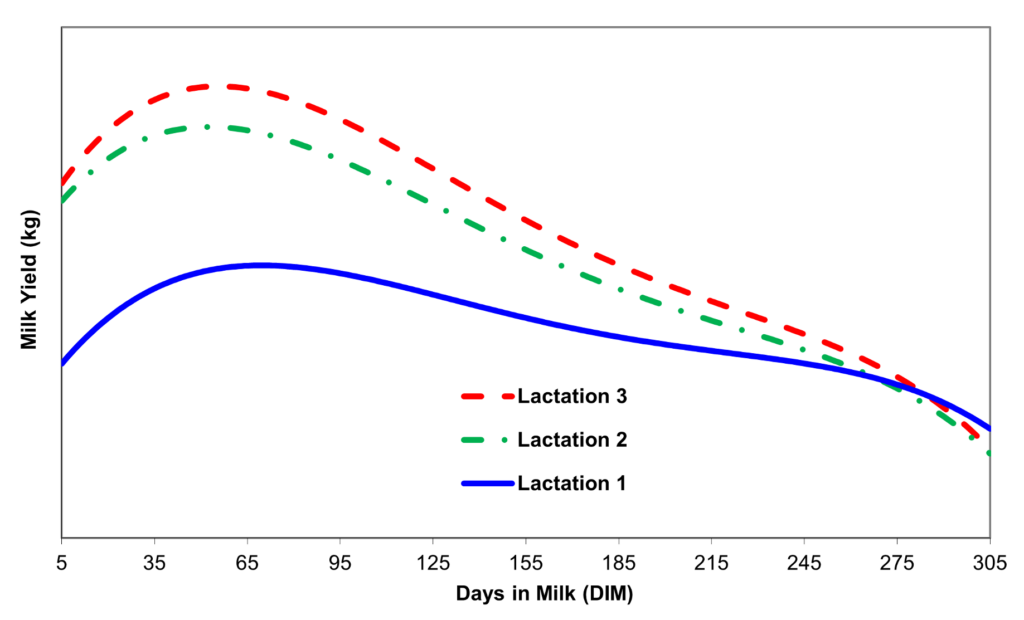
SDCT does take a higher level of management to keep mastitis and SCC levels low. Maintaining proper hygiene in the milking parlor is a critical factor to SDCT success. Understanding the pathogen load on your dairy is also very important.
Molecular diagnostic tools can give producers more granular answers about the types of pathogens that are present in udder infections, versus just knowing that there’s some inflammation process going on due to infections, which is the answer that SCC scores provide. qPCR testing is an efficient and accurate way to quickly determine pathogen type and gram-positive and gram-negative strains.
Testing and herd surveillance
Since mastitis can cause significant economic losses year over year, dairy producers should monitor SCC regularly and the milking team should check cows’ milk and udders during their milking routine. Fast, accurate diagnostic results are needed to identify the mastitis-causing pathogen(s) so that the dairy producer and veterinarian can quickly develop an action plan to minimize loss of milk, duration of treatments, and spread of disease to the rest of the herd.
Once mastitis has been identified in a cow’s quarters, it is important to test the milk because different categories of pathogens require different management strategies. Several diagnostic testing options are available. It’s best to consider the needs of your dairy farm, and then work with your herd veterinarian to develop a mastitis control program that fits your specific operation.
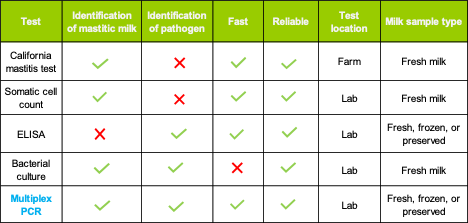
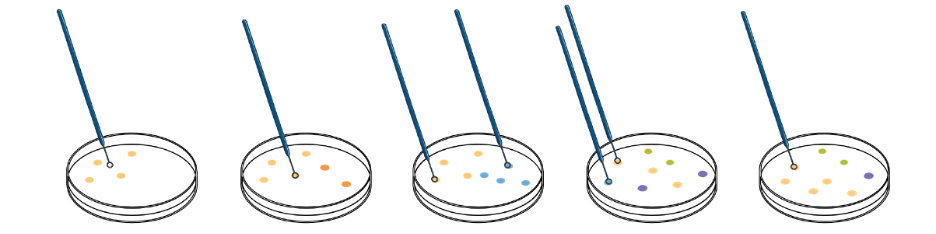
Bacterial culture has traditionally been used in many parts of the world for the diagnosis of mastitis infections. However, culturing can take up to 10 days. When the diagnostician views the culture plate, it may be overgrown with other bacteria, making it difficult to recognize the specific pathogen that is being looked for.
With culture, it’s possible for a few pathogens to grow large colonies that dominate the dish, prohibiting the growth of other pathogens. Unfortunately, fast-growing pathogens aren’t necessarily the most important or the only pathogens to target. Knowing and understanding the full pathogen load is often critical to resolving a high mastitis infection rate in the herd. Mixed infections of two or more mastitis pathogens have been detected more commonly by qPCR than by conventional culture [4].
Advantages of qPCR Testing for Mastitis Detection
About 40% of clinically abnormal milk samples tested by traditional bacterial culture come back with a “no growth” result, often leaving dairy producers right where they started—with a mastitic cow and no diagnosis for accurate treatment.
Real-time qPCR technology detects pathogens by targeting a pathogen-specific (bacterial or viral) nucleic acid sequence within a sample containing nucleic acid from an animal. It essentially detects pathogens by amplifying small amounts of DNA in samples that contain live or dead cells. This also means qPCR can show a history of infection even if it has been resolved. The high sensitivity of this technology enables detection of pathogens even before clinical signs might present themselves, thus allowing for quicker action to separate and quarantine sick animals while keeping the rest of the herd healthy.
Due to its sensitivity and specificity, qPCR detects more pathogens per sample and more minor pathogens compared to culture. PCR provides clear and objective results, which allow veterinarians and producers to make informed decisions to create targeted treatments that can minimize the use of antibiotics. Results from qPCR are usually available the same day and provide a highly accurate positive or negative outcome with low levels of false-positive or false-negative results.
qPCR also offers dairy producers more flexibility to store milk samples until they can get them to a laboratory and samples can be pooled for more cost-effective screening. qPCR can be used with individual cow samples or bulk milk samples, which can be fresh, frozen, or preserved. With culture, milk samples must be fresh.
qPCR more accurately detects mastitis pathogens
qPCR kits, such as the Applied Biosystems™ VetMAX™ MastiType Multi Combo Kit, offer laboratory results in 2.5–3 hours, enabling same-day results even for Mycoplasma species.
The kit is designed to detect 15 mastitis-causing bacteria and an antibiotic resistance gene with just one diagnostic test. It facilitates reliable results with individual cow samples or bulk milk samples, which can be fresh, frozen, or preserved.
VetMAX MastiType multiplex qPCR kits accurately detect mastitis-causing pathogens, allowing the veterinarian to recommend the appropriate treatment protocol and use antibiotics only when needed.
Laboratories can also benefit from the VetMAX kit’s fast and convenient workflow using the Applied Biosystems™ MagMAX™ CORE nucleic acid extraction system. It also uses Applied Biosystems™ QuantStudio™ 5 real-time PCR instrumentation, which employs cloud-based software solutions.
Learn more about our full mastitis workflow solutions.
References
- Rollin E et al. (2015) The cost of clinical mastitis in the first 30 days of lactation: an economic modeling tool. Prev Vet Med. 122(3):257–264. doi:10.1016/j.prevetmed.2015.11.006.
- Bovine diagnostics: How much does mastitis cost dairy producers annually? (2023). https://www.thecattlesite.com/focus/thermo-fisher-scientific/2335/bovine-diagnostics-how-much-does-mastitis-cost-dairy-producers-annually.
- Fidèle K et al. (2021) Comparing blanket vs. selective dry cow treatment approaches for elimination and prevention of intramammary infections during the dry period: a systematic review and meta-analysis. Front Vet Sci. 8:688450. doi:10.3389/fvets.2021.688450.
- Keane OM et al. (2013) Increased detection of mastitis pathogens by real-time PCR compared to bacterial culture. Vet Rec. 173(11):268. doi: 10.1136/vr.101598.
For Veterinary Use Only. For In Vitro Use Only. Regulatory requirements vary by country, products may not be available in your geographical area.




