



Bacterial Isolates from On-Farm Acidified Milk Samples
By Neil Anderson - Animal Health and Welfare/ OMAFRA. Several Ontario producers acidify colostrum, raw milk or milk replacer to reduce bacterial load and to store these at ambient temperature for free-access feeding.The success of acidification as a disinfectant may vary with contact time, the bacterium of interest, pH or other factors. We were curious about what might be growing in pails or barrels of acidified milk on Ontario farms. We visited 24 farms (dairy-cattle and dairy-goat operations), collected acidified milk from 48 containers, and submitted it for bacterial culture. Here are some of our findings.
Figures 1, 2 and 3 summarize data. From 48 on-farm samples, we found 74% were in the recommended pH range of 4.0-4.5. Nine were in the range of 4.6-4.7 and three were in the range 3.6-3.8. On aerobic bacterial culture, the majority of samples (31/48=65%) had less than 10,000 colony-forming units per millilitre (cfu/mL) of milk. Of milk samples in pH range 4.0-4.5, 68% had no growth of coliforms. Environmental Staphylococcus and Streptococcus species were cultured from 54% of the samples and coliforms from 35%. Two of 21 (9.5%) whole milk or colostrum samples yielded Staphylococcus aureus positive cultures with 1-500 cfu/ml. The two samples had contact times of 1 and 7 hours. None of the 21 yielded Streptococcus agalactiae.
Figure 1 shows the results reported as no growth, less than 500 colony-forming units per mL (cfu/mL) of milk, 600-1000 cfu/mL, 1100-5000 cfu/mL and greater than 5000 cfu/mL. The graph shows the number of samples that fell within those ranges for three bacterial species - Staphylococcus, Streptococcus and coliforms.
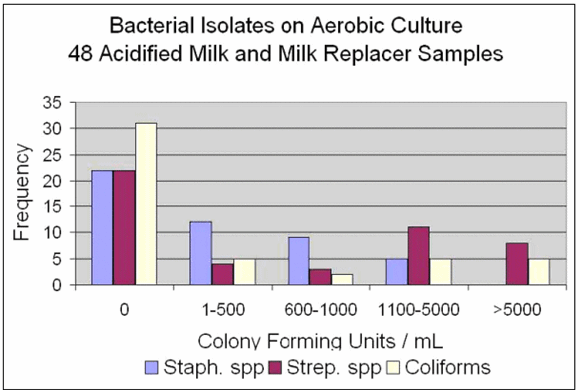
Figure 2 shows the frequency of "no growth" and "positive" coliform cultures and the contact time with acid for the milk samples collected on the farms. For example, 5 samples had one hour contact time and of those, 3 had no growth and 2 were culture positive.
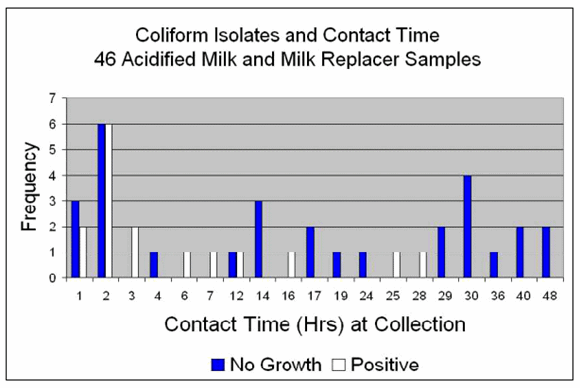
Contact with acid at the time of sample collection ranged from one to 48 hours for the bacterial cultures.
"No growth" for coliform bacteria was more frequent in samples with contact ?12 hours (83%) vs. samples ?7 hours (45%).
Figure 3 shows the frequency of "no growth" and "positive" coliform cultures and the pH of the samples for milk collected on the farms. The graph shows seven samples with pH 4.2. Four of those had no growth and three were culture positive.
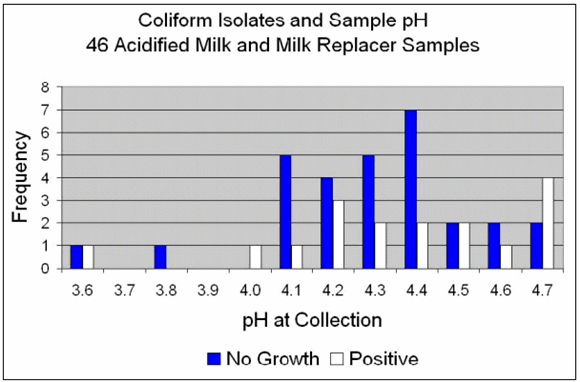
Milk samples ranged in pH from 3.6 to 4.7. The frequency of "no growth" samples for coliform bacteria was greater for pH?4.5 (68%) vs. samples ?4.6 (44%).
The frequency of "no growth" and "positive" cultures for coliforms and Streptococcus species were similar for milk replacer and milk/colostrum samples. However, milk/colostrum samples yielded "no growth" for Staphylococcus species more frequently than milk replacer samples (68% vs. 27%, respectively).
Sources of bacteria in milk for calves may include intramammary, environmental contamination (manure, bedding) at harvesting or contaminated equipment (harvesting, storing, mixing, and feeding). Milk replacer powder is not sterile and water for preparation may be contaminated. For the 48 milk samples in our observational study, the source or degree of bacterial contamination prior to acidification is unknown. Although milk or milk replacer samples generally have unacceptable levels of bacteria when stored for several hours at ambient temperatures, acidified samples from this study yielded low levels on bacterial culture. Colostrum Culture Goals
- Total bacterial count < 100,000 cfu/ml
- Fecal coliforms (lactose positive) < 10,000
- Other gram negative bacteria < 50,000
- Streptococcus agalactiae 0
- Streptococcus non-agalactiae < 50,000
- Coagulase positive Staphylococcus 0
- Coagulase negative Staphylococcus < 50,000
- Others 0 (Mycoplasma bovis, Salmonella)
McGuirk S. 2003
Acknowledgements
Miss Jennifer Garner, OMAFRA 2006 summer-experience student, collected milk samples and survey data at 24 farms. Dr. Anna Bashiri, Mastitis Research Laboratory, Department of Population Medicine, Ontario Veterinary College, University of Guelph, provided laboratory services. Twenty-four Ontario dairy producers welcomed us to their farms to study their free-access feeding systems.
May 2007


