



Milking Practices Recommended to Assure Milk Quality and Prevent Mastitis
By G.M. Jones, Professor of Dairy Science and Extension Dairy Scientist, Milk Quality & Milking Management, Virginia Tech. Table of Contents
Table of ContentsIntroduction
Pre-Milking
Milking
Post-Milking
Summary
References
Introduction
The establishment of mastitis infections is related to conditions that expose the teat end to bacteria (e.g., contaminated teatcup liners, common wash or dry cloths, milkers' hands, dirt or manure in dirty free stalls, muddy environment) and to situations that make it easier for these bacteria to penetrate the teat canal (e.g., squawking or slipping teatcup liners, flooded milk tubes or claws). They travel into the mammary gland where the infection causes an inflammatory response that can cause destruction of milk-secreting cells and release of leukocytes or somatic cells. The bacteria that usually cause mastitis are: Staphylococcus aureus, Streptococcus agalactiae, environmental streptococci, and coliforms. The most successful mastitis control programs concentrate on identifying and eliminating those conditions that expose the teat end to bacteria, assist their penetration through the teat canal, or interfere with the body's immune system. Also they regularly monitor the herd's mastitis status.All of the bacteria listed above can be minimized by proper milking technique, combined with a properly designed and maintained milking system, and environmental conditions that allow cows to remain clean, dry, and comfortable. To minimize mastitis problems and to milk cows more effectively, attention must be paid to cow preparation, stimulation of milk let-down, and procedures used to apply or remove teat cups. From 1962-65, scientists with the National Institute for Research in Dairying in Reading, England, conducted two large field experiments involving 29 herds and 2200 cows and found that a pre-milking hygiene routine of disinfectant udder wash, individual towels, disinfecting rubber gloves worn by milkers, and teat dipping reduced new infections by 44%. In addition to these practices, pasteurization of teatcup clusters with hot water (185 degrees for 5 seconds) reduced new infections by 58%. The general goals for most herds should be to recover all of the milk that cows are bred and fed to produce in as short a period of time as necessary while minimizing effects on udder health and milk composition. However, many dairy farms pay too little attention to the importance of proper milking practices and routine.
Pre-Milking Procedures
Stimulation Causes Milk Let-downMilk is produced throughout the day by milk secreting cells (alveoli) located deep within the udder (they resemble bunches of grapes). About 60% of this milk is stored until milking within the alveoli and small ducts that drain the alveoli. The remainder is stored in large ducts and udder cistern. For complete, fast milk-out, the cow must be stimulated to let down her milk. Sensitive receptors for stimulating milk let-down are located in the teat skin. After stimulation of these receptors, a signal is sent to the brain, and the pituitary gland releases a hormone, oxytocin, into the blood. Oxytocin travels to the udder and causes contraction of the muscle fibers (or myoepithelial cells) that surround the alveoli. Contraction forces milk into the large ducts and udder cistern where the milking machine then can remove the milk. A normal milk let-down can be interrupted if cows become frightened or excited either before or during milking. Environmental stresses reduce milk yield, increase milking time, and may cause mastitis. Milking routines need to be consistent from day to day and milker to milker and cows need to be handled gently. On many farms, the time delay from first stimulation until units are applied (to be referred to as stimulation time) ranges from 2 to 6 minutes. These long stimulation times can contribute to lower milk production and fat percentage, slower milking, and higher somatic cell counts or mastitis problems. Maximum oxytocin concentration in the blood occurs 1 minute after the beginning of stimulation. Within 1.5 to 2 minutes, oxytocin concentration drops dramatically to half the maximal concentration and let-down is reduced.
For most effective milk let-down, attach the units after 1-minute stimulation. Use a clock to time yourself and, if necessary, change your routine. A 3-5 minute stimulation time has been shown to reduce milk production by 16%. In contrast, many milkers spend only 5 to 8 seconds to wash and dry a cow's teats. A 5-8-second hand wash with running water was shown to be ineffective in stimulating milk let-down. By comparison, hand stimulation of the teats for 30 seconds increased milk production by 26 to 33%. One recent study found that 5 powerful squirts of foremilk should be removed, followed by scrubbing the teat five times for 20 seconds.
Studies at the University of Minnesota indicate that stimulation times of less than 1 minute or more than 2 minutes are associated with the development of severe, chronic lesions on teat ends. Get in the habit of attaching the milking machine at 1 minute from the time that stimulation begins.
Don't prepare too many cows in advance. Milking units should be attached within 1.5 minutes. If cows are dirty and you spend more time on any one cow, don't proceed to the next cow until after the unit is attached to the first cow.
Milkers Should Wear Gloves
S. aureus has been isolated from noses and hands of dairy workers which could serve as sources of mastitis infections for cows and heifers. English workers included rubber gloves in their hygiene routine, which reduced new infections by 44%. Packs of 100 latex or vinyl disposable gloves cost from $.11 to $.19 per glove. Dairy workers should consider using disposable gloves that would be disinfected throughout milking and thrown out at the end of every milking, especially if herds are infected with S. aureus.
Use Strip Cup
Stripping 4-5 squirts of milk from each quarter is beneficial because it allows you to detect early stages of clinical mastitis, removes foremilk which may have high bacteria counts, may serve as the primary stimulus for milk let-down, and assists in reducing mastitis. During foremilk stripping, wipe dry dirt off the teat with the hand.
In USDA (Beltsville) studies, stripping before washing and drying the udder reduced the incidence of new udder infections from 18 to 7%. Stripping after udder preparation was less effective. Foremilk stripping assists in the early detection of clinical mastitis. Look for the presence of flakes, clots or stringiness, or watery secretions. Hard quarters that are warm or enlarged provide an early warning that the cow has clinical mastitis and that her milk should not be added to the bulk tank.
Use a strip cup with a black surface. The detection of a watery secretion may indicate that a problem is developing. Take the affected cow's temperature. Many mastitis organisms are air-borne and live outside the udder. When a strip cup is not used, milk often is squirted onto the feet and legs of the cow being prepared, or the adjacent cow. Infections can be transferred via the feet and legs to the bedding, whereby an uninfected cow may use the stall next and become infected. The bacteria can thrive in dirty, wet stalls. If milk is squirted on the parlor floor, flush it away with a water hose. Do not squirt foremilk on hands.
Wash Teats with a Sanitizing Solution
Scrub teats and teat ends thoroughly with a paper towel or direct a stream of sanitizing solution on the teats and wash by hand. Do not wet the udder beyond 2 to 3 inches above the teats. Remove all dirt and manure from teats, including back sides of teats that are most difficult to reach. Use only as much water as needed to cleanse the teat. The more water you use, the harder it is to dry off teats. Long hair on udders should be removed regularly as dirt and manure may adhere to the udder and make it more difficult to properly clean and dry teats.
Do not wash teats with a common sponge or cloth. A bucket of sanitizing solution does not kill all bacteria present on a common udder cloth or sponge. Thus, these bacteria are transferred from infected cows to clean cows. Use a paper or cloth towel on one cow only.
Pre-dipping
Pre-dipping, where cows' teats are dipped in germicidal teat dip prior to milking, has become an important part of the pre-milking preparation. It can serve as a substitute for washing teats with sanitizer. However, dirty teats must be cleaned before pre-dipping. Dip should remain on the teat approximately 30 seconds before it is dried-off with a paper or cloth towel. Drying is important to avoid increased teat dip residues in milk. Pre-dip will destroy those microorganisms which contaminate the teat skin between milkings. Pre-dipping has reduced new cases of mastitis caused by coliforms and environmental streptococci. Also, be sure to continue to teat dip after milking.
The same teat dip can be used as a pre- or post-dip, but two different dippers should be used. Dippers are preferred over sprayers unless use of sprayers results in adequate coverage of backsides of teats. If iodine teat dips are used, low iodophor concentrations (0.5% or less) should be used since 1% iodophor has resulted in a mild increase in milk iodine content. Dips should contain up to 10-14% skin conditioner (e.g., glycerol, lanolin) for prevention of chapping.
The effectiveness of pre-milking teat dipping is reduced by wet and dirty cows. Such cows should receive a first wash of the teat with either a wet paper towel or hand washing, but don't wet the udder. Pre-dip after the dirt has been washed away.
Cleaning and Sanitizing Gel
Scientists at Louisiana State University tested the effectiveness of a gel containing 0.5% iodophor and glycerol when applied to teats for 30 seconds and dried with a single service paper towel. When compared to washing with water followed by iodine pre-dip, gel treatment reduced somatic cell counts, bacteria counts, infection rate, and cases of clinical mastitis. The gel resulted in shorter prep times and greater parlor throughput.
Dry Teats Thoroughly
Teatcups should be applied to clean, dry teats. When water droplets on the udder drain toward the teat end, they pick up bacteria. This dirty water can be sucked inside the teatcup and raise the bacteria count. Admitting large amounts of air into the teatcup causes milk droplets to move backward and up the milk tube. It's similar to a fine aerosol spray, causing milk droplets and any bacteria inside the teatcup liner (contaminated liners or dirty water on teat ends) to impact against the teat end.
Bacteria may strike the teat ends with enough force to cause them to enter the teat canal. These impacts are caused by vacuum loss created when units are attached, when units are removed without shutting off the vacuum, by squawking teatcups, machine stripping, or unit falloff.
Dry wet teats and udders thoroughly. Leave no water on the teat or udder. Use single service paper or cloth towels. Do not use any towel on two cows as infection may spread. Do not fold the cloth towel over and use the back side for a second cow. Cloth towels do a better job of getting teats dry, are preferred by milkers, and may be cheaper, but they must be laundered before next use.
Milking
Milking RoutineEstablish a uniform work routine that results in smooth cow flow, uses good milking practices, and makes the best use of your time.
- Prep cow 1: forestrip; wash and/or pre-dip.
- Follow the same procedure with cow 2 and perhaps a 3rd cow.
- Return to cow 1, dry with single-service towel and attach milking unit.
- Dry and attach milking units to cows 2 and 3.
- Go to next 2-3 cows and follow same routine used for cows 1-3.
Adjust and align teatcups so the quarters milk out properly. As the teatcup is attached, hold the short milk tube down over the claw ferrule to minimize the amount of air that rushes in. Position the teat inside the teatcup so that milk flow is not impeded. Use a support arm for milker hoses or claws. If a teatcup should squawk, adjust it as soon as possible. Vacuum loss contributes to the reverse movement of milk droplets inside the liner and bacteria can impact against the teat end and enter the teat canal. When replacing teatcup liners, be sure the liner does not become twisted inside the shell. Also, make sure there isnºt any water between the shell and the liner. If milking only three teats, twist the fourth teatcup and lay it over the claw so that it does not admit air. If plugs are used, wash them properly after milking.
Machine Stripping Isn't Necessary
In most cases, if cows are properly prepared and milked with a good milking system, machine stripping is not necessary. If practiced, machine stripping should take no more than 15 to 20 seconds. Massage each quarter in a gentle downward motion with one hand while applying a slight downward pressure on the claw with the other hand. Do not exert enough pressure to cause air to leak around the mouthpiece or to cause the teatcup to slip.
Research conducted by USDA at Beltsville showed that 1.6 minutes of machine stripping resulted in higher somatic cell counts and more milk being discarded because of mastitis. Nebraska studies suggested that milking techniques were improved, working conditions were more relaxed, and labor efficiency was higher by eliminating stripping.
Removing the Milking Unit from the Cow
Remove the units from cows by shutting off the vacuum. Sounds simple enough, right? After several seconds, the teat cups will slide off gently. Do not pull teatcups off before the vacuum has been shut off. This kind of removal causes a stress on the teat and air slippage is detrimental. Wait until all four quarters are milked out and then remove all teatcups at the same time by shutting off the vacuum at the claw. Faster-milking quarters may be milked out 1 to 2 minutes before other quarters. Leave them alone unless they begin squawking or slipping, in which case the squawking teatcups should be removed immediately by pinching off the short milk tube of the teatcup liner.
Also, do not remove a teatcup by pinching off the short milk hose and twisting the liner around the ferrule on the claw. In most cases, air will enter the mouthpiece and cause vacuum loss inside other teatcups. It is less damaging to leave that teatcup on for another 10 to 20 seconds or even a minute.
Remove the unit as soon as the cow is milked out. Do not squeeze the short milk tube at the bottom of the liner to detect milk flow. The constriction causes vacuum fluctuation at the teat end. Most cows will milk out in 4 to 6 minutes. Studies at the University of Minnesota indicate that condition of the teat end deteriorates when units remain on the cow for more than 5 minutes. You cannot remove all of the milk from the udder. Approximately 10-15% of the milk remains in the udder as residual milk.
If you aren't able to remove the units as soon as the cow is milked out, maybe you are attempting to use too many units. Without automatic detachers, most operators cannot use more than two units properly in a stanchion or milking barn and three units in a parlor. Automatic detachers have increased labor efficiency, but many Virginia dairymen with automatic detachers have two operators milking in double-6 and double-8 parlors. They probably don't need the second person. With automatic detachers, sound milking procedures are still most important.
Some older cows may not milk out completely with automatic detachers. Some operators say they have to reattach the milkers to about 10% of the cows, but it's usually the same cows. Minimize vacuum loss as the units are reattached. Remove them properly.
Post-milking
Post-milking Teat DippingAccording to the National Council, teat dipping is the single most effective practice for reducing mastitis infections, especially by contagious bacteria. Dipping all teats after each milking has a greater impact on reduction of milk somatic cell counts and increased milk yields than any other milking practice. Effective teat dips should destroy microorganisms present on teats at the end of milking. This prevents bacteria from establishing a colony at the teat end or in teat lesions where they could penetrate the teat canal and establish an infection. In addition, a good teat dip should leave a residue on the teat so the antimicrobial action is still present when the cow lies down in a free stall or any other place where sanitary conditions are less than ideal. Effective teat dips have reduced new udder infections by 50 to 90%. As soon as the units are removed, dip teats in a sanitizing solution that is intended for teat dipping. Do not use multipurpose cleaners or sanitizers. Squawking teatcups, vacuum losses, contaminated teatcup liners, and wet-cow milking contaminate teat skin with bacteria that can cause mastitis. Cracks and lesions on the skin surface are additional problems because they harbor bacteria and make sanitizing more difficult.
Teat Dipping Methods. Cover the entire teat with dip. Don't forget the back sides, especially if you use a spray. Occasionally, check the far side of the rear teats as soon as they've been dipped. Is coverage complete? In many cases, the back sides are hardly touched. Dip at least the bottom half of all teats after every milking, using an FDA approved teat dip. Most effective coverage may be attained by dipping, compared to spraying. A dipper makes covering the whole teat easier. With spraying, more attention is required to assure good coverage. It is easy to miss the back half of the teat, especially with hand, bottle-type sprayers that spray from the side. Cracks and lesions on the teat harbor mastitis-causing bacteria that thrive when teat dip is not used or coverage is incomplete.
According to Pankey (University of Vermont), the following management options can improve effectiveness of teat sanitation:
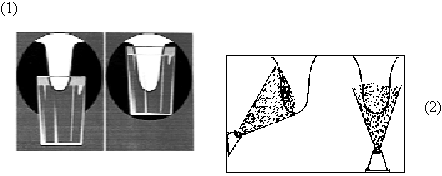
Figure 1. Two frequent problems in teat dip applications:
(1) Failure to dip most of the teat when using a dip cup, and (2) Failure to spray the back side of the teat.
Precautions
Teat dip must be kept clean and sanitary. Never pour contents of a dipping cup back into the jug or storage container at the end of milking, thus contaminating unused teat dip. Discard any remaining teat dip. Rinse dippers or cups with water and allow to dry. Sanitize them at least weekly.
Discard teat dip contaminated with manure or dirt.
Follow manufacturer 's recommendations for storage, usually in a dark, cool, dry, clean place. Storage temperatures above 90 degrees or below freezing can deactivate the germicidal agent or cause separation and result in teat end damage. During cold weather, store teat dips where they will not freeze.
Cold weather. Exposure to extremely cold weather after teat dipping may cause chapped or frozen teat ends. Make sure that teat dip contains a skin conditioner (a maximum of 10-14%), such as glycerin or lanolin. Keep the cows in the parlor for at least 1 minute contact time and dry teats with a single-service paper towel before they are released from the milking area, or hold them in the milking area until teat dip has dried. Idaho research showed no detrimental effect when teat dipping and blotting dry continued with temperatures under 10 degrees. Teat dipping can be discontinued when temperatures are much lower and cows must be turned out. Protect milking parlor return alleys from wind to reduce effects of low wind-chill temperatures.
Use only a product that has been developed and tested as a teat dip. If teat dip is transferred to a smaller container (e.g., gallon bottle), make sure that it is clearly identifiable so that milkers do not mistakenly use other type sanitizers as teat dips. Non-teat-dip sanitizers can be harmful to teats. Don't allow any chance for other sanitizing solutions to be mistaken for teat dip. Be sure the sanitizer is a teat dip, and, when bottles of sanitizing solution are refilled, make sure all bottles are clearly marked and every milker understands what solution is in each bottle, dipper, or container.
Teat dipping gives little control over contamination that occurs between milkings since the disinfectant activity persists only for a short period of time after application. Consequently, post-milking teat dipping is not as effective against coliform infections as it is against contagious bacteria and may only be partially effective against mastitis caused by environmental streptococci bacteria.
Although teat dipping is extremely effective in the prevention of new mastitis infections, teat dipping will have little impact on the duration of existing infections. Mastitis infections persist for months or even longer. The impact of teat dipping on frequency of mastitis infections in a herd can be increased by dry-cow therapy of all cows plus culling of problem cows with chronically high somatic cell counts or cows receiving numerous treatments for mastitis.
Some germicides used in teat dips may be mildly irritating to cows. Herdsmen experiencing such problems should discontinue use of that specific teat dip and try another product. The benefits of teat dipping on mastitis control are too great to dispense with teat dipping.
When are other times to use teat dip? In addition to pre- and post-milking use of teat dip, teats also should be dipped in an effective teat dip after administration of an antibiotic at drying off. Other successful uses have included dipping teats prior to collecting aseptic milk samples for culturing and before administration of an antibiotic into the udder.
Use of Barrier Teat Dips
Barrier teat dips generally contain germicides, skin conditioners and protective film so that the teat end is sealed from mastitis-causing bacteria. Research has shown that barrier teat dips do inhibit bacterial multiplication on the teat skin under the film. To properly evaluate a barrier teat dip, dairy farmers should request data where the specific barrier teat dip has been compared to a control post-milking teat dip under natural exposure conditions. Post-milking teat germicidal dips have limited effect on incidence of new mastitis infections caused by environmental streptococci and coliform exposures between milkings, long after the bacteriocidal activity has diminished. In addition to being effective against these environmental pathogens, barrier teat dips must be effective against contagious pathogens spread as a result of milking. For example, research has shown that a .55% chlorhexidine barrier teat dip was comparable to 1% iodophor. There was no difference in rates of new infections due to streptococci and S. aureus or total clinical cases. The barrier teat dip was more effective against E. coli but less effective against Pseudomonas spp. and Serratia spp. Also a barrier teat dip containing chlorous acid-chlorine dioxide was equivalent to iodine against environmental pathogens in a natural exposure study and reduced S. aureus and Streptococcus agalactiae in experimental challenge study (where a bacteria-containing broth is dipped on teats and then teats are dipped with teat dip to determine effective bacterial kill). Although successful for cows in lactation, studies with barrier teat dips have found none that will persist for 7-14 days during early and late dry period.
Dipping or Backflushing Milking Units
When removed from a cow, most teatcup liners are contaminated with bacteria. For many herds, dipping of teatcup clusters in a sanitizing solution is not convenient. In herds where Staphylococcus aureus infections are present, dipping or backflushing units is a must, especially if the S. aureus infected cows can't be segregated from other cows. Rinse teatcups in lukewarm water followed by a rinse in a hot sanitizing solution. Hang teat cups for several minutes so they can drain and dry. Teatcup liners must be dry before they are placed on the next cow. The solution must be kept relatively hot and changed as soon as it starts to change color or becomes lukewarm. Automatic backflush units are available and may be beneficial in certain dairy herds.
Milk S. aureus Infected or Mastitis-Treated Cows Separately or Last Cows with clinical mastitis, S. aureus infected cows or cows which have been treated with antibiotics should be milked last, or milked with separate milkers or milking units equipped with backflush to avoid spread of infection via contaminated teatcup liners. Segregation of S. aureus infected cows has been proven to significantly reduce the prevalence of S. aureus mastitis and bulk tank somatic cell counts.
Summary
Maximum milk let-down requires 25 to 30 seconds of stimulation per cow, followed by attaching milkers 1 minute after preparation was started.
Strip 4-5 squirts of milk from each quarter before preparing the cow.
Wash teats with only as much water as necessary.
Pre-dip and allow 30 seconds contact time.
Dry teats thoroughly.
Check your timing. Attach milking units within 1-1.5 minute after stimulation is started.
Milking units must be properly aligned on the udder.
Don't over-milk. Remove milking units by shutting off the vacuum.
Dip teats.
Backflush units or segregate cows with S. aureus infections.
Selected References
Burmeister, J. E., L. K. Fox, D. D. Hancock, C. C. Gay, J. M. Gay, S. M. Parish, and J. W. Tyler. 1995. Survey of dairy managers in the Pacific Northwest identifying factors associated with teat chapping. J. Dairy Sci. 78:2073-2082.
Fox, L. K., and R. J. Norell. 1994. Staphylococcus aureus colonization of teat skin as affected by postmilking teat treatment when exposed to cold and windy conditions. J. Dairy Sci. 77:2281-2288.
Grove, T. M. and G. M. Jones. 1992. Use of an enzyme-linked immunosorbent assay to monitor the control of Staphylococcus aureus mastitis. J. Dairy Sci. 75:423-434.
Ingawa, K. H., R. W. Adkinson, and R. H. Gough. 1992. Evaluation of a gel teat cleaning and sanitizing compound for premilking hygiene. J. Dairy Sci. 75:1224-1232.
Kingwill, R. G. 1981. The NIRD-CVL mastitis control method. Pages 24-39 in Mastitis Control and Herd Management, Tech. Bull. 4, National Institute for Research in Dairying, Reading, England.
Nickerson, S. C., and R. L. Boddie. 1995. Efficacy studies on barrier teat dips. Pages 38-47 in Proc. 34th Annu. Mtng., National Mastitis Council, Madison, WI.
Pankey, J. W. 1992. Practical milking tips: Pre- and post-dipping. Pages 94-100 in Proc. 31st Annu. Mtng., National Mastitis Council, Madison, WI.
Rasmussen, M. D., E. S. Frimer, D. M. Galton, and L. G. Petersson. 1992. The influence of pre-milking teat preparation and attachment delay on milk yield and milking performance. J. Dairy Sci. 75:2131-2141.
Rasmussen, M. D., D. M. Galton, and L. G. Petersson. 1991. Effects of pre-milking teat preparation on spores of anaerobes, bacteria, and iodine residues in milk. J. Dairy Sci. 74:2472-2478.
Timms, L. L. 1995. Positioning barrier teat dips in the dry period. Pages 48-55 in Proc. 34th Annu. Mtng, National Mastitis Council, Madison, WI.
Wilson, D. A., R. N. Gonzalez, and P. M. Sears. 1995. Segregation or use of separate milking units for cows infected with Staphylococcus aureus: Effects on prevalence of infection and bulk tank somatic cell count. J. Dairy Sci. 78:2083-2085.
June 1998



