Working to Limit Infectious Bovine Rhinotracheitis and Effect on Cattle Reproduction
Timing of Infectious Bovine Rhinotracheitis vaccinations needs to coincide with heifer cycles if high conception rates are to be achieved, writes Jillian Fain, University of Georgia.Important Facts about IBR
Infectious Bovine Rhinotracheitis (IBR), commonly referred to as "Rednose," is a disease resulting from bovine herpesvirus type 1 (BHV-1), writes Mrs Fain, Department of Animal and Dairy Science.
The difficulty in managing this disease without vaccination is complicated by the ability of field strains to remain latent until reactivated, generally by a stressful event.
Examples of stress include but are not limited to poor nutrition, shipping, illness or overcrowding. Once reactivated, there is viral replication through both the respiratory and reproductive systems. The subclinical nature of many IBR cases makes the identification of the disease within a herd difficult unless blood testing is performed routinely in open herds.
Summary
- IBR is a viral infection that can lead to reproductive inefficiency and failure in cattle.
- Vaccines, regardless of type, must be handled and administered according to label directions to maximize vaccine utility and minimize potential adverse effects.
- Try to vaccinate heifers at least two cycles prior to breeding in order to maximize conception rates.
- If you are going to vaccinate with an MLV during pregnancy, you must have previously vaccinated with same vaccine before breeding – though this does NOT remove the complete risk of abortion.
- Never use an MLV in purchased, pregnant animals with unknown vaccination history.
- Submit tissues to a diagnostic laboratory from abortions to monitor for BHV-1 and other contagious diseases.
Decisions related to vaccination for IBR are often related to its respiratory and not reproductive form. The detriment of the disease, as well as the positive benefits vaccination can have on a reproductive program, needs to be on the forefront of a producer’s herd health program.
Respiratory symptoms resulting from IBR are generally non-lethal; however, the immunosuppression that coincides with the body’s fight with the disease can lead to secondary infections that may result in mortality. Bacterial members of the family Pasteurellaceae often cause the secondary infections, with the most common infector being bacterial pneumonia. The majority of actual "mortality" associated with IBR is a result of the inability to maintain pregnancies and the resulting culling loss of reproductively inefficient animals that may occur.
Abortion rates associated with IBR have been reported to range from 5 to 60 percent in herds without a vaccination program. Historically, BHV-1 is the most diagnosed viral cause of abortion in North American cattle (Table 1). Current research amongst five veterinary diagnostic laboratories (Iowa, California, Washington, Minnesota, South Dakota) also reaffirm the incidence rate as well as pattern in the United States over a 10-year period from 2000-2010 (Table 2).
Carried in the bloodstream via white blood cells, the virus is able to gain access to the placenta and eventually the developing fetus. After arriving at the placental tissue, BHV-1 causes the condition placentitis. Placentitis is a general term used to describe any inflammation of the placenta.
Placentitis is often accompanied by vasodilation (blood vessel expansion), which increases the permeability and blood flow to the placenta and thereby the developing fetus. The virus is particularly drawn to the fetus as it prefers actively growing tissue. The time from infection to ultimate abortion varies between animals, although once the virus begins replication in the developing fetus, death can occur in as little as 24 hours.
Abortion may not occur immediately after death as the fetus often goes through a period of autolysis. Though embryonic death can occur early on, most loss is associated with abortions that occur in animals greater than five months in gestation. Subsequent necrosis of the fetal liver and placental tissue are often identified post-mortem. Additionally, the expelled fetus will have a dark red coloration due to the blockage of hemoglobin. The tricky part in identifying an IBR-related abortion is that often the abortion occurs without any other clinical signs of IBR. Clinical symptoms vary depending on the severity of infection as well as the tissues that are infected.
The term "Rednose" was attributed to this disease as damage to the nasal passage and trachea can lead to inflammation of the nostrils and muzzle. Inflammation is often accompanied by an elevated temperature (>104°F). The respiratory damage may also lead to difficult, either labored or rapid, respiration and nasal discharge. There may be general lack of animal thriftiness as well with loss of appetite and depression. While observations of the aborted fetus and placenta often cannot lead to conclusive diagnosis of IBR, there are diagnostic tools that can help, including fluorescent antibody, immunohistochemistry, polymerase chain reaction or virus isolation.
Reproductive inefficiencies resulting from IBR are not limited to abortions. There are additional long-lasting impacts on fertility with IBR directly impacting ovarian structures. With the movement of the virus via the blood, it has direct connection with the ovaries.
There is well-documented evidence that IBR causes temporary necrotic oophoritis or necrosis of the ovary most often characterized by severe lesions on the corpus luteum (CL). Additionally, subsequent developing follicles are susceptible to degeneration. These follicles, if able to ovulate, have the potential to release oocytes of reduced viability as a result of viral interaction.
Clinical observations have noted congested surface vessels, hemorrhagic tissues within the CL, and both fibrinous fluid and clotted blood within follicles. The ovary appears to be most susceptible to damage post-estrus, when a new CL is forming.
Using a Modified Live Vaccine to Address IBR
There are numerous advantages of using a modified live vaccine (MLV) instead of a killed or inactivated vaccine. To some producers, use of an MLV offers greater security and ease to their vaccination program. The security comes from its ability to initiate a stronger, more rapid cell-mediated immune response (replication) while the ease is a result of the relatively long duration of immunity without booster vaccinations.
In addition, the MLV reduces injection site reactions and lesions, which are commonly a consequence of the adjuvants associated with killed vaccines. Certain disadvantages must also be considered when dealing with the live, though attenuated, form of the viral organism itself. Attenuated simply means that the viral particles are live and will grow in the recipient of the vaccination but have been altered to a less pathogenic form.
Fragility of the live vaccine is the primary consideration; therefore, proper handling is essential to maintain viability. These products also have a short shelf life following re-constitution with rapid reduction in their viability seen in as few as one to two hours following rehydration. Another issue with the MLVs is their potential to induce abortion in pregnant animals. This concern travels not only directly to the vaccinated dam but also to the dam that is being nursed by a vaccinated calf. Some of these concerns were alleviated with relabeling by the USDA in 2004.
Prior to 2004, the use of an MLV in a pregnant animal was relatively unheard of. The risk for late-term abortions was high and the label statement by the USDA read "do not use in pregnant cows or in calves nursing pregnant cows." In these animals, their killed counterparts were the only option. Label revisions made in 2004 allowed the use of an MLV in pregnant animals as long as the cow was previously exposed to the vaccine pre-breeding, generally within the past 12 months.
Heifer directions for administration might vary from cows, as they may require additional exposure. The adherence to the vaccination during pregnancy as the second exposure to the live organism is essential. Abortion rates where the pregnant animal was naïve to the vaccine have been very high in studies performed between 2000 and 2009 (both before and after USDA label changes).
Historic data has indicated how vaccination with the MLV could potentially impact the fertility of an animal, while some research questions the true efficacy of using an MLV in a pregnant animal, regardless of previous exposure.
IBR Vaccination and Breeding
Timing of vaccinations is much easier to address when working cows versus youngstock. Time and labor can be a limiting factor in the general practices surrounding a heifer management program. Consolidating handling times and thus labor costs is a common goal. One such consolidation may include vaccinating around breeding time potentially as part of a synchronization program.
Aside from labor minimization, there are additional agendas to pre-breeding vaccination. This vaccination is generally performed in an effort to prevent infections that could ultimately affect that animal’s ability to sustain pregnancy.
When following this agenda, it is important to know that research indicates that vaccine administration needs to be a minimum of one month prior to breeding. The reasons behind this time frame are two-fold. First and foremost, this allows for adequate response of the immune system for proper protection before initiating a breeding program. Secondly, the research demonstrates that MLVs administered too close to breeding can have detrimental effects on an animal’s reproductive success.
There are similarities in the physiology of how an animal reacts to a vaccine, whether that animal is a pregnant cow or a 14-month-old pre-breeding heifer. This similarity is that previous exposure to the vaccine appears to mitigate the negative reproductive side effects associated with the vaccination. There is an overall reduction in the inflammatory response if the animal is not naïve to the vaccine. Therefore, if vaccination closer to breeding is necessary, the animal is less likely to have negative reproductive consequences if she has been previously exposed to the vaccine.
Additionally, use of a killed vaccine (non-replicating) as opposed to an MLV in a naïve heifer appears to reduce the risk of abnormal cycling associated with vaccination at breeding, which ultimately lends higher conception rates. Abnormal cycles are seen in less than 15 percent of heifers when vaccinated with a killed vaccine one week prior to breeding, regardless of previous exposure to the vaccine.
This is compared with more than 35 percent of animals cycling abnormally when a modified live was administered as a single dose a week prior to breeding. Despite these outcomes, it is imperative to remember that effective immunization appears to be maximized when vaccination occurs a minimum of three weeks prior to breeding with either a killed or modified live vaccine.
The impact of vaccination on cyclicity shares some similarities with naturally occurring viral symptoms. The vaccination elicits an inflammatory response. This inflammatory response is the result of the body’s reaction to either the live organism of an MLV or an adjuvant used in a killed vaccine.
The most negative impacts on the reproductive system have been observed with MLV administration inducing oophoritis, which is an analogous condition to that caused by the naturally occurring virus. The inflammation ultimately disrupts the hormone profile of the estrous cycle. Previous studies have indicated ovarian tissue with high levels of inflammatory cells, hemorrhage and necrosis as a result of an MLV administration.
This battle that occurs at the site of inflammation is similar to what occurs in mammary tissue as a result of a mammary infection. Figure 1 illustrates the resulting alteration in the estrogen hormone profile as a result of MLV exposure in a study conducted by Perry et al. (2013).
Damage to ovarian structures resulted in abnormal estrous cycles in animals vaccinated with a single injection of MLV eight days prior to breeding, as shown in Figure 2. Abnormal estrous cycles were defined as those less than 15 days in length or in which blood progesterone never rose above 1ng/mL following breeding, which is the concentration indicative of a functional CL.
Vaccinating for IBR with an MLV in Pregnant Cattle
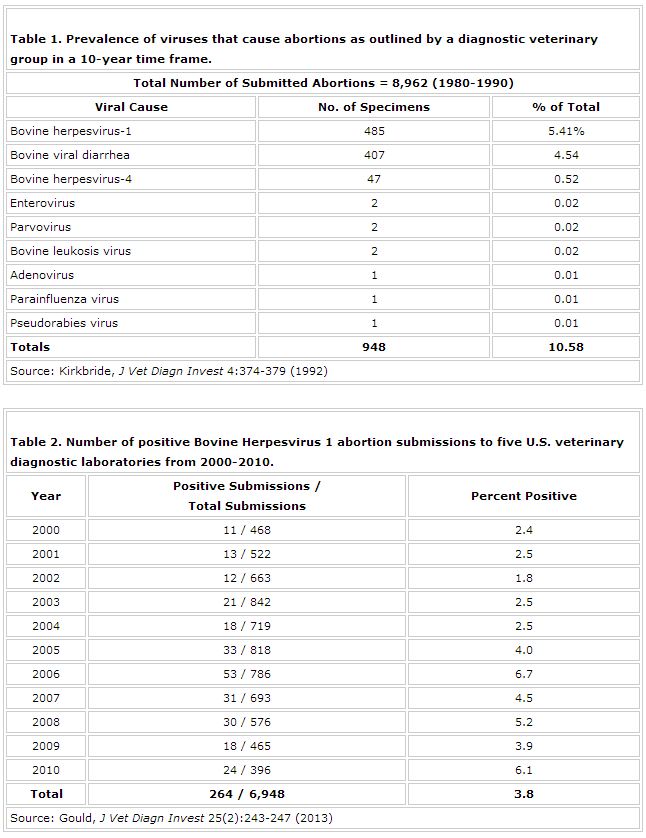
Data from the Colorado State Veterinary Diagnostic Laboratory have indicated that their number of aborted fetuses that tested positive for IBR increased markedly in 2008-2009 compared to their 2002-2003 data (Figure 3).
This represents more than a 10-fold increase in the number of IBR-related abortions from before and after the 2004 USDA MLV label change. With few submitted vaccination records, the increase recorded by the Colorado State laboratory may be a result of improper use of the MLV.
Many of these abortions were potentially a direct result of vaccine administrators not adhering to label directions or failing to vaccinate prior to breeding. The rise, though not as extreme, is also substantiated by the Gould data previously discussed, in which there was a rise in the number of IBR abortions after 2004.
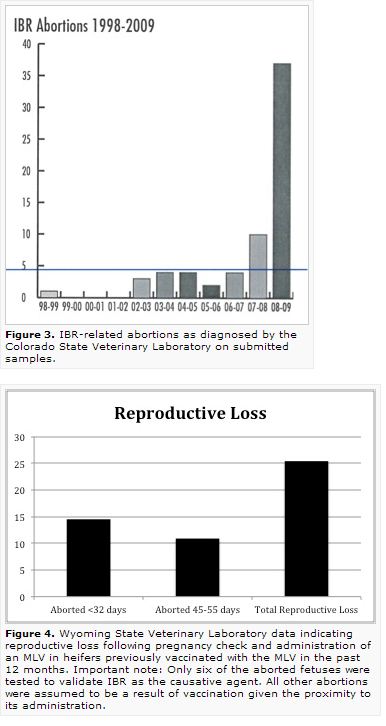
Other research published in the Journal of American Veterinary Medical Association (JAVMA) from the Wyoming State Veterinary Laboratory (Figure 4), suggests that abortion can occur even when animals were previously vaccinated. Cattle in that trial (55 Angus-cross heifers) were vaccinated with an MLV respiratory five-way three different times pre-breeding (last time in May) and were revaccinated with the same MLV in December at approximately seven months of gestation when pregnancy was confirmed by ultrasonography. The MLV label used in the study indicated, like others, that the product was NOT to be used in pregnant cows unless they had also been vaccinated within the past 12 months. The Wyoming operation adhered to label guidelines.
Of the 55 heifers re-vaccinated in December, eight had observed abortions with six being submitted for diagnostic work that confirmed IBR in fetal tissue. These abortions occurred between 45 and 55 days after vaccination. Of the remaining 47 animals, 41 calved. The remaining six animals had an assumed loss less than 32 days following pregnancy examination and vaccination. If all losses could be attributed definitively to the vaccine, total reproductive loss would be 25 percent. The six definitive cases still represent a loss of 11 percent.
Other diagnostic labs have similarly confirmed abortion losses of 5-25 percent following MLV vaccinations. Additionally, one large production unit (>5,000 head) has reported a change in abortion rates following discontinuation of a BHV-1 MLV product as advised by their veterinarian, R. Corbett (Figure 5). The producer indicated that all label directions were followed with pre-breeding vaccination; however, it must be noted that the study was not conducted under controlled conditions. The trend with all of these observations is the heifer groups are predominantly involved and that those later in gestation (> 7 months) are at a higher risk rate.
Conclusions
There might be future help in identifying the source of the new abortion cases on an operation. There is a current inability to link the IBR strain in the aborted fetus to a field or vaccinated strain. Previous work by Kennedy and Richards (1964) indicates that abortions occurring 23-52 days following vaccination may be the result of the vaccine, whereas when naturally challenged, abortions occurred some 2.5 months later after replication of IBR in the respiratory tract. The more rapid abortion in vaccinated animals is likely a result of the increased number of organisms when compared with natural exposure.
Producers should approach using MLVs in pregnant animals with caution until more research is published with data to support or disprove current label directions. If choosing to use an MLV in pregnant animals, always strictly adhere to label directions on storage, handling and administration. The consequences of an abortion and the resulting potential effect on fertility could prove costly. Consult with a veterinarian or vaccine manufacturers when making these kinds of animal health decisions.