Partnering during bluetongue outbreak to offer diagnostic solutions
The Pirbright Institute is home to the OIE World Organisation for Animal Health Reference Laboratory for bluetongue virus (BTV). The team at Pirbright is responsible for receiving samples for diagnosis from the UK and around the world and responding to disease outbreaks. Diagnosing the correct strain of the virus is critically important, so the appropriate vaccine, if available, can be identified and administered in order for the spread of disease to be brought under control as quickly as possible.

Dr. Carrie Batten is Head of the Non-Vesicular Disease Reference Laboratory and leads the OIE Reference Laboratory for bluetongue. Work within her group primarily focuses on the diagnosis of bluetongue virus and other veterinary viruses and validates and applies new technologies for the detection and characterization of important livestock disease agents. The Reference Laboratory at The Pirbright Institute also monitors the global patterns of BTV to recognize new emerging viral lineages that threaten the UK and Europe.
The current BTV situation in Europe
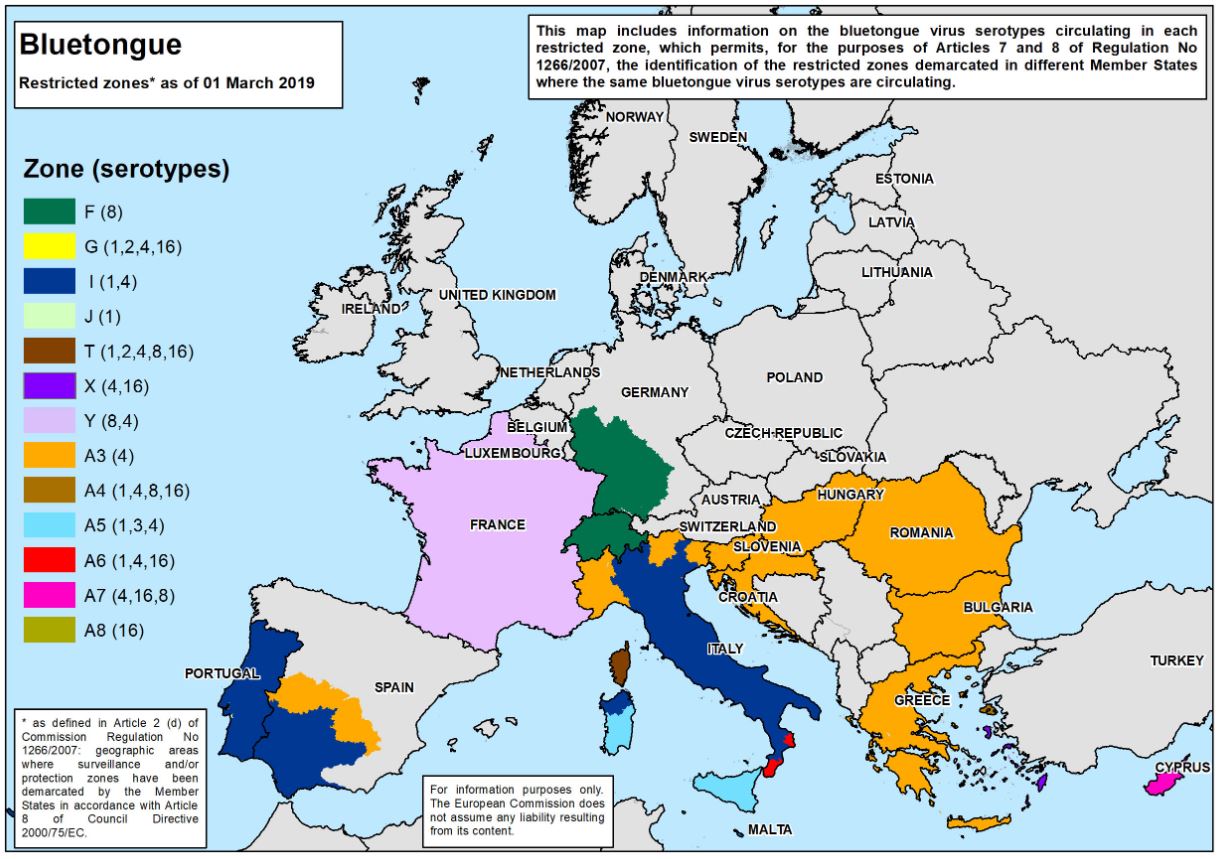
A significant outbreak of bluetongue virus serotype 8 (BTV-8) occurred in northern Europe from 2006 to 2009. It was brought under control through regulation of animal movements and mass vaccinations across many EU member states. However, in 2015 BTV-8 re-emerged in France. In 2017, bluetongue virus serotype 4 (BTV-4) also appeared in France, and the two viruses are currently circulating together. In late 2018, BTV-8 was detected in Switzerland and southwest Germany, which has expanded the disease area.
"To further confuse matters, there is a BTV-4 strain causing outbreaks in the Balkans and Italy, so there is a real mix of BTV circulating, said Dr. Carrie Batten, Head of the Non-Vesicular Disease Reference Laboratory and OIE Reference Laboratory lead for BTV at The Pirbright Institute. “In addition, we have BTV-3 circulating in Tunisia, Sicily and Sardinia which emerged in 2016 and 2017, and recently BTV-2 was detected in Tunisia. There are now concerns BTV-2 will also move further north into Europe following the same route as BTV-3.”
The difficulty with having more than one virus serotype circulating in common areas, such as in France, is their ability for reassortment. Reassortment occurs when the genome segments of two different virus strains mix in the same cell, and a virus with a new combination of genome segments emerges.
“In a host that’s infected with two serotypes, reassortment can lead to a virus potentially emerging that has increased virulence and other properties that were not characteristics of the parental strains,” said Dr. Batten.
“The newly created virus is not a new serotype; it is a new variant. It’s a bit like what happens with influenza. The genome segments can swap around. For example, a virus could have every segment from BTV-4 except for one segment it’s inherited from BTV-8,” added Dr. Batten.
 BTV’s presence year-to-year
BTV’s presence year-to-year
Bluetongue virus is transmitted by midges of the genus Culicoides (insect vector) among susceptible ruminants, primarily sheep, cattle and goats and certain wildlife species such as deer. Since midges are the only significant natural transmitters of BTV, distribution and prevalence of the disease is governed by ecological factors such as high rainfall, temperature and humidity. The high midge season is normally March to September.
The fluctuating impact of BTV is difficult to explain. In the Mediterranean, BTV has been circulating for years.
“Historically, researchers working on BTV thought that bluetongue transmission was limited to the Culicoides imicola midge,” said Dr. Batten. “Culicoides imicola is a Mediterranean species. Whereas now, it’s clearly been shown that BTV can be transmitted by other species of Culicoides, such as those found in northern Europe.”
Compared to several years ago, Culicoides populations have expanded into new regions. If the insect vector is there to transmit the disease, the disease can go there, she said.
“Why did this particular strain of BTV-8 come back?’ That’s the question that everybody’s asking,” noted Dr. Batten. “It’s been hypothesized that it was circulating in wildlife or that there was low-level circulation of the virus existing under the limits of detectable surveillance. The fact that BTV-8 got here in the first place clearly shows that the doors are open for other serotypes to follow.”
At present, inactivated vaccines are only available against serotypes BTV-1, BTV-4 and BTV-8. Vaccines are being used to facilitate animal movements and to try to control the disease. Producers need to vaccinate against the serotypes circulating in their geographical area.
BTV’s impact on animal health
The clinical signs in animals seen with infection of different BTV serotypes vary because each serotype has different properties. For example, the BTV-8 strain that was circulating from 2006 to 2009 had quite high morbidity in both cattle and sheep. The current circulating BTV-8 strain in France has shown milder clinical signs in susceptible animals. However, there have been recent reports of calves being born small, blind and dying within a few days. These calves have been shown by PCR to be positive for BTV-8, suggesting transplacental transmission.
Cattle are the reservoir host for BTV. They can become infected and exhibit a high level of viremia that lasts a long time, but they don’t necessarily show outward signs of infection.
With sheep, more classical clinical signs are seen, such as fever, lameness and edema, but it tends to impact the less hardy English breeds more. The UK’s BTV-naïve population could be severely impacted.
Partnering with Thermo Fisher Scientific to make diagnostic tests available
In 2007, the Pirbright team developed a series of 24 bluetongue detection tests targeting VP2. These serotyping tests are used in a second step to serotype the strain. The BTV serotyping tests were based on Pirbright’s virus collection of 3,500 isolates from all over the world.
“Working together with Thermo Fisher, we enabled laboratories in countries across Europe to use these tests to detect and serotype a positive sample within a couple of hours,” Dr. Batten said.
“We couldn’t have done that ourselves; we needed a commercial partner to take it on and develop and distribute tests and manage logistics,” she noted. “People are still using the VetMAX products, and they work very well. If you’ve got more than one serotype circulating in one area, it’s important to determine the serotypes quickly without running a lot of different tests. This kit allows you to rapidly define what serotypes you are dealing with.”
Preventing the spread to BTV-free countries
In BTV-free countries, the goal is to try to prevent the introduction of BTV through imported, infected animals but at the same time make sure trading is facilitated. The EU has established guidelines about how animals can be moved from a BTV-restricted zone into a BTV-free country using vaccination.
For example, if a UK farmer wants to import animals from France, the animals would have to be vaccinated against both BTV-4 and BTV-8 and wouldn’t be able to be moved to the UK until 60 days after the completion of the vaccination course. The goal is to establish immunity in the animals before they are shipped. Upon arrival in the UK, they go directly to the farm, and the imported animals are put under restrictions. Then seven to 10 days after arrival, the animals are sampled and tested by PCR to ensure that they are BTV-negative. Once the test comes back BTV-negative, the restrictions are lifted.
“In the UK, we’ve got a passive surveillance system in place, where veterinarians are encouraged to look for clinical signs of BTV – a notifiable disease – and report it to Defra (Department for Environment, Food & Rural Affairs),” Dr. Batten explained. “Samples will be collected and tested in the laboratory using PCR or serology to confirm or negate the presence of BTV.”
“Additionally, annual surveillance is conducted in the UK to ensure there’s been no incursion of bluetongue by means other than importation. Because we’re an island and relatively close to France where infection is widespread, there is obvious concern that infected midges could cross the channel and infect animals in the UK,” explained Dr. Batten. “We do targeted PCR surveillance along England’s southeast coast, which is performed at the end of midge season to identify any evidence of infection during the vector season.”
Dr. Batten said a lot of effort goes into communicating BTV information and risk levels to farmers, so they can decide if it’s appropriate to vaccinate their herd or flock and also highlight the risks of importing animals from BTV affected areas.
“If we were to suddenly see a very high density of bluetongue infection in the animals that border the coastline, we would help heighten awareness, and farmers would probably have more of an inclination to vaccinate,” she said.
BTV diagnostic monitoring program
Pirbright uses real-time RT-PCR and ELISA to detect group-specific responses. Real-time RT-PCR is also used for post-import testing, frontline diagnosis and confirmation of positive samples that have been submitted to the OIE Reference Laboratory.
“We always use real-time RT-PCR to determine if BTV specific nucleic acid is present. If there is, it will trigger a whole array of other tests, for example serotype specific real-time RT-PCR tests to determine BTV serotype,” Dr. Batten explained. “In some instances, we look at the serological status of animals, and that will be done using ELISA, which detects BTV-specific antibodies, not serotype-specific antibodies.”
Using the Thermo Fisher KingFisher Flex Purification System, they are conducting automated nucleic acid extraction from samples using magnetic beads. This delivers nucleic acid within about an hour which can then be used in real-time RT-PCR to give an overall diagnostic result within 2.5 hours.
Reference laboratories and diagnostic companies working together
“At the time of the BTV outbreak in 2006, no one would have guessed it was BTV-8,” said Dr. Batten. “Everybody predicted that if this was going to be a bluetongue incursion, it would’ve been one of the five that were currently circulating in the Mediterranean at the time.”
The risk for a new serotype or strain to emerge in a region is always present. Early awareness is the key. The network of Reference Laboratories helps provide this through the diagnostic and monitoring capabilities they possess, and the regular sampling carried out.
The OIE Reference Laboratories are sent samples from countries all over the world, giving them a global view of what serotypes and strains are currently circulating. The network of BTV labs, including the OIE network and the EU Reference Laboratory (EURL) network, is communicating and distributing samples to ensure that diagnostic tests are fit for purpose and the information about those strains is available to the community.
“Our role as an OIE laboratory is to ensure that when there is a new BTV threat, we can very quickly check the strain and if needed rapidly develop a new test,” noted Dr. Batten. “That is where I hope Thermo Fisher Scientific and other diagnostic companies would want to work with the Reference Laboratories to ensure that the best tools are out there for whatever the threat is at that point in time.”
50+ years of expertise. Applied. Visit our webpage.
For veterinary use only. For in vitro use Only. Regulatory requirements vary by country; products may not be available in your geographic area.
TheCattleSite News Desk