



Beef cattle implants
By Dale ZoBell, C. Kim Chapman and Kevin Heaton, Utah State University Carl Birkelo, Schering-Plough Animal Health.Introduction
Mechanism of action
Implant performance
Implant use
Recommendations of correct sanitation procedures when implanting
Implants commercially available
Other considerations
Implanting strategies
Ration considerations
Side effects
Conclusions
References
Introduction
Growth promoting implants have been used extensively in beef production for over 30 years. Significant changes in implants and implanting strategies have occurred. Prior to 1987, available implants were estrogenic agents which metabolically enhanced nutrient use to enhance growth. These products improved feed efficiency 5-10 percent and daily gains from 5-15 percent. In 1987, the androgenic (tissue building) agent, trenbolone acetate, was approved for use in growth promoting implants. This compound had an additive effect with existing estrogenic implants. The androgenic implant enhanced muscle growth and added an additional 2-3 percent to the feed efficiency and 3-5 percent to the daily gains. The return on implant investment varies, but only in rare situations do implants return less than $5 per $1 spent. Implants are available for all cattle except calves less than 45 days old and most breeding cattle. Proper scheduling and use of implants should return in excess of $10 per $1 spent.Today, implants have become almost designer products with varied doses and combinations of estrogenic and/or androgenic agents. While implants tend to be most effective in feed yards, implanting strategies have been effectively applied to other beef production situations. The growth promoting implants approved for use in the United States are extremely safe. They are safe not only for the cattle, but for producers who use the products and for the consumers who consume the beef produced from implanted cattle. There is no withdrawal time for any of the approved implants available in the United States.
Mechanism of action
Cattle must have adequate nutrition before implants can positively influence feed efficiency and gain. The greatest response to implants tends to be observed in older cattle, such as yearlings, near peak periods of lean tissue deposition. Typically these would be yearling cattle consuming high levels of high energy feed.Estrogenic implants increase the circulating levels of somatotropin (ST) and insulin-like growth factor-1 (IGF-1). Both of these substances are produced by the animal and have a marked effect on how nutrients are used by the animal to produce muscle, bone, and fat. The approved androgenic agent, trenbolone acetate (TBA), does not seem to stimulate the production of ST, but does significantly increase the circulating levels of IGF-1 and decreases the normal loss of muscle tissue in sedentary animals. The implant response is associated with nutrients available and the level of implant growth promotant circulating in the animal.
When growth promoting implants are first placed in the animal, there is a rapid release of hormone from the implant. The level of growth promotant being released from the implant will begin to fall after a few days, but will remain above an effective growth stimulating level "threshold" for a varying length of time depending on the pharmaceutical design of the implant and the quality of technique used when administering the implant placement. Reimplanting, the administration of an additional implant, is usually scheduled to coincide with the declining level of circulating implant growth promotant, but always above threshold. The optimum re-implant time is referred to as the re-implant window. For maximum benefit, it is important to maintain the level of implant growth promotant above threshold throughout the ownership of the stocker or feeder animal. The length of time an implant releases growth promotant varies from approximately 75 days for Ralgro® to the manufacturer’s estimated 200 days for Compudose®. The improvements in rates of gain appear to follow the declining level of growth promotant released from an implant. Therefore, the highest rates of gain can be expected during the first part of the release period.
Because implant growth promotants interact with the production of hormones by the animal, they have not been recommended or approved for use in breeding cattle or calves less than 45 days of age as hormone production in these animals has not yet started.
Implant performance
The estrogenic implants approved for use in suckling calves will improve weaning weights 3-5 percent. Similar performance improvements can be seen in pastured stocker cattle when the base gain is above 1.5 pounds per day.Previously implanted cattle are of concern to cattle buyers who take advantage of compensatory gain potential of cattle. Producers who do not implant suckling calves or stocker cattle should receive a premium equivalent to the loss of production they would have achieved had they implanted.
In feeder cattle, estrogenic growth promoting implants improve feed efficiency and gain 5-15 percent. Implants which include TBA can provide an additional 3-5 percent improvement in feed efficiency and daily gain. A properly designed re-implant program can sustain implant associated improved performance beyond the payout that would be expected for a single implant. For estrogenic implants used in yearling cattle fed typical feedlot rations, at least a $5 return above the cost of the implant can be expected for each $1 price of a bushel of corn. Adding TBA to an estrogen implant system will return an additional $2 above the cost of the implant for each $1 price of a bushel of corn. For example, if corn costs $3 per bushel, an estrogenic implant would return approximately $15.
Limited data suggest cull cows respond to implants at or above the level of younger feeder animals, especially to TBA. Most cull cows are not fed long enough to consider a re-implanting program.
Implant use
Regulations governing the use of implants are set by the U.S. Food and Drug Administration (FDA). Always read and follow the manufacturer’s directions before implanting.The only approved location for implant administration is the middle third of the back side of the ear. All implants must be located within this area (Figure 1). This location has been chosen because the ear is removed and discarded at time of slaughter, reducing any food supply risk. If part of the ear has been lost because of frost bite or injury, the implant should be placed in the last third of the ear.
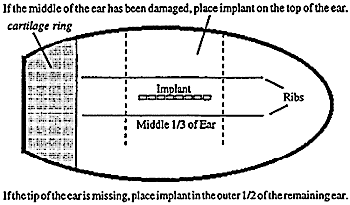 Figure 1. |
Recommendations of correct sanitation procedures when implanting
The major sanitation problem to overcome is the removal of fecal material (manure) from the implant area, containing bacteria, by simple cost effective procedures. These procedures physically remove the manure, dirt and bacteria, and also apply a disinfectant solution to the implant injection site surface of the ear. Only with a bacteria free implant injection site and a clean, disinfected implant needle can consistent abscess free implanting results be obtained.Coliform bacteria are normal inhabitants of an animal’s digestive tract, and during handling and processing can be spread to the hair and skin surfaces of cattle. As cattle are gathered and driven to the processing area, and are processed through cattle facilities, fecal material can be deposited on various parts of the body simply by close contact. This is unavoidable but can be reduced by less crowding and limiting the time spent in a confined area. Fecal material becomes a problem when injections are made through contaminated skin and hair. Implants, other pharmaceuticals and vaccines can be made partially or totally ineffective when bacteria are carried into the injection site by the needle; logically, the larger the needle gauge the more debris that can be carried into the injection site. The animal’s body provides the perfect environment for opportunistic microorganisms to grow and multiply. As a consequence of the microbial growth and subsequent infection, abscesses are formed and can result in the possible reduced efficacy of the animal health product administered.
As expected, growth implant efficacy and return on investment decreases as abscess severity increases. Cattle having complete abscesses where all implant pellets are expelled from the injection site have similar performance to cattle not given a growth implant. Typically, with complete abscesses the implant pellets are expelled within three to five days following implanting. Perhaps the largest unknown is the decrease of growth implant efficacy that occurs when slight to moderate abscesses develop which limit drug absorption by tissues surrounding the implant.
Kansas State University research (Spire et al., 1999) reported average daily gains were decreased 8.9% (3.18 versus 2.92 pounds) and feed efficiency decreased 8.5% (5.62versus 6.14 pounds of feed per pound of gain) by abscessed growth implants. It is imperative employees receive instruction on proper implant technique and sanitation procedures. Some cattle operations continue to have a great deal of variation in proper implanting rates because they fail to recognize that dirty ears can cause abscesses, and are unwilling to implement cleaning procedures.
A number of years ago a method called “scrape, brush and disinfect” was introduced to raise the awareness of ear sanitation prior to implanting by cattle processing personnel. In this method an initial assessment of ear surface cleanliness is made. If the ear is clean and dry, the implant is administered. If the ear is dirty (wet or dry), a dulled steak knife is used to scrape foreign material from the ear prior to brushing the ear with disinfectant solution and implant administration. If the ear is wet, but contains no foreign material, it is brushed and disinfectant solution and the implant given. The type of brush used to clean and disinfect the ears is two-sided with brass bristles on one side and nylon bristles on the other. When the brush is not in use, it is placed in a small bucket of disinfectant solution.
Choice of disinfectant is extremely important for effective control against the major microorganisms that can cause growth implant abscesses. One of the most effective and safest disinfectants is Nolvasan (chlorhexidine acetate) and is licensed by the EPA. It has a rapid bactericidal antiseptic action with sustained residual activity for as long as two days. It also retains anti-microbial activity in the presence of organic matter. (Taken from Fort Dodge Animal Health Technical Bulletin FDP 310 8/99.)
Some feed yards coat the cleaned implanting needle with an approved, non-irritating antibiotic between animals as an additional safeguard to help prevent implant site infections. Visit with your veterinarian about the selection, dilution, and use of a disinfectant.
Developing a light touch and slightly rotating the needle when implanting is the best defense against cartilage embedment. A properly placed implant will be slightly movable.
Missing or bunching of implant pellets can be avoided by carefully restraining the animal and slowly withdrawing the implant needle as the implant is being administered. Implant guns and needles are available from the companies that manufacture growth promoting implants. All implants can be effectively administered with the implant gun designed for the associated implant. It is important to visually inspect and physically palpate the implant site after the implant is administered to ensure the implant is properly placed and all the pellets in the pelleted implants are properly aligned. As part of the inspection, the implant needle opening should be closed by pressing down on the hole. Most of the problems with implant guns can be avoided by closely following the manufacturer’s directions.
Routine inspection of implant and vaccine sites should be done every time animals are handled through a chute and at periodic quality audits performed at packing houses. A practical and consistent inspection can be accomplished on each animal that enters the hospital pen.
Implants commercially available
Currently available implants vary in type and concentration of active anabolic compound.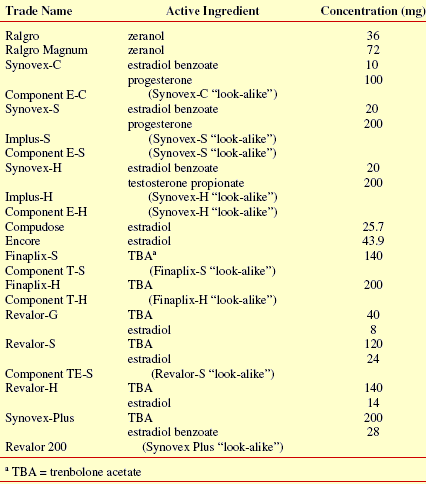

Tables 3 through 5 list implants approved for use in suckling calves, stocker and feedlot cattle respectively. Estimates of the effective life for each implant are based on conclusions drawn by various scientists from published field trials as well as company claims based on explant and blood assay research. As you can see, determination of the effective life of an implant is an imprecise process at best. That should not be a surprise given the numerous factors involved (e.g. implanting technique). Estimates are useful for recommending an optimum time for reimplanting. However, it is not appropriate to use them to compare implants. Amount of additional weight gained is the bottom line, not how long it took an implant to produce the added weight. Effective life is not the only factor affecting how much additional weight is gained.

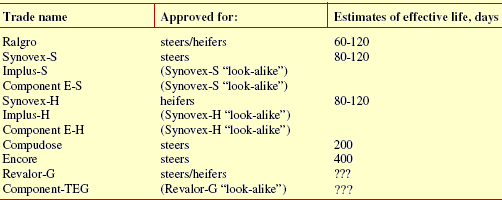
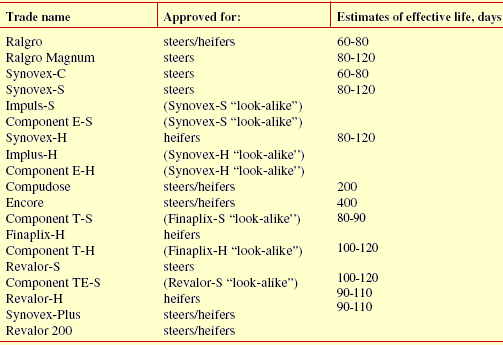
Other considerations
Implanting heifers intended to enter the breeding herd is controversial. The mixed results from research trials suggest detailed management considerations must be adhered to before considering an implant program for replacement heifers. Implant once and not younger than the age directed. Highlights of these considerations include selecting an implant approved for use in replacement heifers, providing adequate nutrition for growth, and leaving adequate time between implanting and breeding. Implanting replacement breeding bull calves is not approved or recommended.Implanting strategies
It is important to implant cattle as soon as practical. In suckling calves, the traditional branding time provides an excellent opportunity to implant and vaccinate most of the calves in the herd. Prior to bull turn out, the preferred procedures include vaccination with subcutaneously administered modified live four way viral and clostridial vaccines, and implanting calves older than 45 days old with a product designed for suckling calves. It is important not to implant replacement heifers intended to be kept for breeding purposes unless strict adherence to manufacturer’s directions are followed and never implant bull calves.At weaning, calves not intended for breeding should be implanted again with a more aggressive implant. The feeder implant can be either an estrogenic implant or a combination estrogenic-trenbolone implant. It appears to be important to finish the feeding period with the most potent implant selected in the implanting program. Therefore, if a combination estrogenic-trenbolone implant is selected as the first implant, it should be used again in subsequent implantings. If an estrogenic implant without trenbolone is selected as the first implant, a similar product or an estrogenic-trenbolone implant can be selected for subsequent implanting.
Reimplant schedules should be developed to reflect the targeted finish date, the historic grade price spreads, the genetic potential of the cattle, and the feeding program available. From the projected finish date, reimplanting should be scheduled by back calculating the payout days of the last implant intended for use.
For example, if 550-pound medium to large frame weaned steer calves enter the feedyard the first of October, an estrogenic product such as Magnum®, Synovex-S®, or Implus-S® can be selected as the initial implant. If the cattle are projected to gain 3 pounds per day and be marketed at 1,100 pounds, the estimated sale date would be the first two weeks of April. Back calculating the 120-day payout of a combination estrogenic-trenbolone implant from the middle of April, reimplanting would be scheduled for he middle to the end of December.
Maintaining implanting schedules can be very difficult, but tremendous performance advantages can be achieved if properly managed. If you have any questions, seek the advice of a qualified feedlot nutritionist or veterinarian.
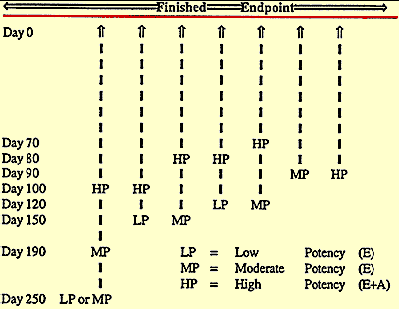 Figure 2. Possible Implant Programs and Use Relative to Days from the Packer |
Ration considerations
Although no special ration considerations are needed for maximal implant performance, it is important to feed a balanced high quality ration. All approved feed additives used in an approved manner are appropriate to consider in a feeding program for implanted cattle. Performance improvements associated with approved feed medications are additive to the expected performance improvements from implants.
Side effects
Heavy carcass weight can be a problem when feeding large frame exotic long yearlings. Typically, implanted cattle will be heavier when finished, if fed for the same amount of time, and with the same quality grade as non-implanted cattle.Poor yield grades have been reported in heifers implanted with combination estrogenic-trenbolone implants and concurrently fed the feed additive melengesterol acetate (MGA). These observations were made in studies designed to evaluate the benefits of a 9 Utah State University Extension is an affirmative action/equal employment opportunity employer and educational organization. We offer our programs to persons regardless of race, color, national origin, sex, religion, age or disability. Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, Robert L. Gilliland, Vice-President and Director, Cooperative Extension Service, Utah State University, Logan, Utah. (EP/08-2000/DF) combination implant. It is likely the heifers were fedtoo long. It is important in any feedlot management program to evaluate cattle near their target finishing date, and market the cattle as soon as they reach the most economical degree of finish.
Poor quality grades can be a problem if implanting schedules are not properly designed to match the age, weight, genetics, and nutritional management of the cattle. It is always important to consider the historic quality grade price spreads at the targeted finishing date. Implanting does have an effect on marbling and grading, depending on the implant strategy employed, and this can affect quality grade. An increase in the buller rate has been reported with the use of some implants. Crushing implants has also been blamed on the increased buller rate in some groups of implanted animals. With the modern implanting tools available today, this problem seems unlikely. The effects of climatic changes, ambient temperature, animal handling, commingling, feed stuffs containing fungal or plant estrogens, and implant technique seem more likely to play a role in these observations.
Vaginal and rectal prolapses have been reported as an implant side effect. If hormones are involved in these occurrences, it is possible additional estrogenic compounds from the feed are also involved. These compounds could come from feed molds or from some classes of feeds such as legumes containing phytoestrogens. Other suspected causes include improper implanting technique or improper implant scheduling.
High tailheads, sunken loins, udder development, and heavy hide weights have also been reported. These problems are generally rare or have minor economic significance when compared to the performance benefit realized from the use of implants.
Conclusions
Using growth promoting implants is one of the most cost effective methods of enhancing cattle gain and efficiency of gain. Implants enhance protein deposition while diminishing fat accretion. Properly designed implant programs should take into account animal age, sex, weight, breed and market objectives. Meat and animal products from cattle implanted with growth promotants are as safe and acceptable as comparable products derived from nonimplanted cattle. For those producers who choose not to implant, there are niche markets that may be developed, providing beef products to individuals who desire this type of beef.
REFERENCES
Hollis, L. C. 1989. Proper management of implant technique in feedlot cattle. Compendium of Continuing Education. Practicing Veterinarian, 11:763-768.
Rains, J., D. Nash. 1990. Implantation: Waste not. Large Animal Veterinarian, Jan-Feb:18-21.
Spire, M. F., D. A. Blasi, J. S. Drouillard, and J. M. Sargeant. 1999. Implant quality assurance: detection of abscessed implants and their effect on feedlot performance of beef heifers.
Kansas State University, Cattlemen’s Day Report, Report of Progress 831:124.
August 2000



