



Managing Milk Fat Depression
The biohydrogenation (BH) theory represents a unifying concept to explain the basis for diet-induced milk fat depression (MFD) where unusual intermediates of ruminal fatty acid BH accumulate in the rumen and eventually reduce milk fat synthesis in the mammary gland, says T. C. Jenkins1 and G. D. Mechor as part of the proceedings from the University of Florida Dairy Extension 20th Symposium.The ‘Biohydrogenation Theory’ of Milk Fat Depression
Under certain dietary situations the rumen environment is altered and a portion of BH occurs via a pathway that produces trans-10, cis-12 CLA and trans-10 18:1 (Figure 1). Bifidobacterium, Propionibacterium, Lactococcus, Streptococcus, and Lactobacillus isolates from other habitats have been reported to produce trans-10, cis- 12-CLA. As these genera occur in the rumen, although generally at rather low numbers, they may contribute to BH and specifically to trans-10, cis-12-CLA formation in the rumen. Propionibacterium, Streptococcus, and Lactobacillus are also more numerous in the rumen with concentrate diets (Jenkins et al., 2008), which would again be consistent with greater trans-10, cis-12 CLA production with concentrate diets. Therefore, dietary situations causing MFD alter the pathways of rumen BH resulting in changes in the specific trans-18:1 and CLA isomers available for uptake by the mammary gland and incorporation into milk fat.
In vivo studies have revealed a vast array of trans-18:1 and CLA isomers present in digesta contents of cattle and sheep (Bauman and Lock, 2006). These in vivo studies were important in showing that many more intermediates exist in ruminal contents than can be accounted for by most accepted pathways of BH. As many as 17 CLA isomers have been identified in ruminal contents taken from cattle (Jenkins et al., 2008). Yet, most published pathways of BH account for the synthesis of only one or two CLA isomers. Much of the attention has been directed at intermediates of linoleic acid BH because it is believed to be the parent compound for most of the CLA isomers found in digesta contents of cattle. A recent study completed at Clemson University incubated a stable isotope of linoleic acid with ruminal contents for 48 h. The results revealed the formation of seven CLA isomers from linoleic acid, including cis-9 trans-11 and trans-10 cis-12 CLA. The other isomers labeled with the isotope were trans-9, trans-11 CLA, cis- 10 trans-12 CLA, cis-9 cis-11 CLA, cis-10 cis-12 CLA, and trans-10 trans-12 CLA.
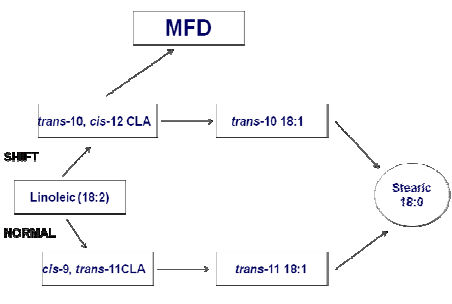
As shown in Figure 1, this ‘trans-10 shift’ in BH pathways, and the associated increase in the trans-10 18:1 content of milk fat, is indicative of the complex changes in ruminal BH pathways characteristic of MFD. Although trans-10 18:1 does not directly inhibit mammary synthesis of milk fat (Lock et al., 2007), it is relatively easy to analyze compared to trans-10, cis-12 CLA and other CLA isomers. Therefore, in general, this fatty acid can serve as a surrogate marker for the type of alterations in rumen BH that characterize diet-induced MFD.
Risk Factors for a Microbial Shift
Rumen Fat Load
Total fatty acid concentration in the rumen can be a key factor contributing to a microbial shift and the elevation of the trans-10, cis-12 CLA isomer. Elevating fatty acid concentration in ruminal contents may cause a number of changes in ruminal fermentation characteristics and microbial population distribution. Ruminal changes are the result of the antimicrobial nature of unsaturated fatty acids, where fatty acids adsorb onto the cell membrane of selected microbial species, and then penetrate into the membrane causing disorganization of phospholipids and eventual cytological damage (Jenkins, 2002). Because some bacterial species are more susceptible than others, the result is a microbial shift in the rumen. The fatty acid-induced microbial shift can disrupt fermentation of carbohydrate digestion causing a drop in the acetate to propionate ratio and possibly a reduction in fiber digestion (Jenkins, 2002). The microbial shift also can redirect the pathways of fatty acid BH causing accumulation of CLA isomers linked to milk fat depression (Bauman and Lock, 2006).
Two factors that affect the antibacterial activity of lipids are fatty acid structure and concentration. Free fatty acids generally disrupt fermentation more than triglycerides and antibacterial activity of free fatty acids can be enhanced by increasing the number of double bonds (Chalupa et al., 1984). Growth of some bacterial species is stimulated by low concentrations of fatty acids, but inhibited at higher concentrations (Maczulak et al., 1981). In attempting to predict ruminal fermentation changes caused by dietary lipid, it is often assumed that the fat load is contributed only by the fat supplement and that FFA concentration is low. Both assumptions can be wrong. Fatty acids from the TMR and forage can significantly contribute to total rumen fat load, for example when animals are consuming immature pasture. Also, FFA concentration may be elevated in some feed ingredients such as whole cottonseed stored in warm, humid conditions (Cooke et al., 2007), or in forages resulting from hydrolytic cleavage of esterified lipids during hay-making (Yang and Fujita, 1997).
The term “rumen unsaturated fatty acid load” (RUFAL) is intended to reflect the total unsaturated fatty acid supply entering the reticulo-rumen each day from feed consumed. As defined, RUFAL accounts for intakes of unsaturated fatty acids from all feed ingredients rather than fatty acid intake coming only from fat supplements. It is hoped that RUFAL will be a better indicator of fermentation disruption in the reticulorumen than relying just on the percentage of fat added to the diet. Past studies show that increasing RUFAL will eventually cause fermentation disruption, which can have negative effects on animal performance. Reduced feed intake and ruminal carbohydrate digestibility can lead to reduced milk production when RUFAL becomes excessive. Another negative consequence of excessive RUFAL is MFD.
RUFAL is calculated as the sum of the three primary unsaturated fatty acids consumed by dairy cattle, namely oleic (C18:1), linoleic (C18:2), and linolenic (C18:3) acids. Average RUFAL from five published studies (Table 2) ranged from 473 g/d for dairy cattle fed no added fat to 696 g/d when cattle were fed fat supplements. The range of RUFAL within diet was 220 to 973 g/d for the control diets and 362 to 1118 g/d for diets with added fat. Much of the RUFAL range could be accounted for by differences in DMI and fatty acid concentrations in the diet DM.
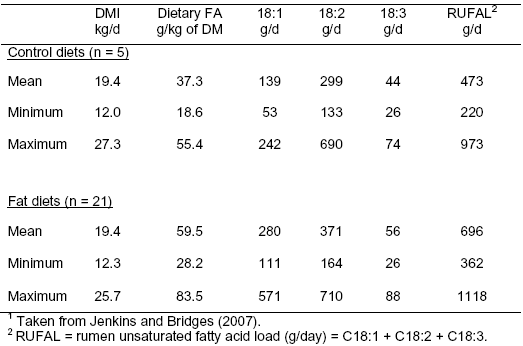
From the data in Table 1, it is clear that the basal diet accounts for a considerable portion of RUFAL. The ratio of RUFAL control/RUFAL for fat diets was 0.68 in Table 2 showing that 68 per cent of RUFAL was contributed by the basal diet ingredients. Fatty acid concentrations in forages and grains typically range from 2 to 4 per cent of DM, but their high intakes expose the rumen microbial population to considerable lipid. Lipid contributions from the basal diet are often overlooked, with much of the focus directed at fatty acid contributions from the fat supplements only. As a result of ignoring basal lipid contributions, variability in animal responses often do not line up with added fat levels.
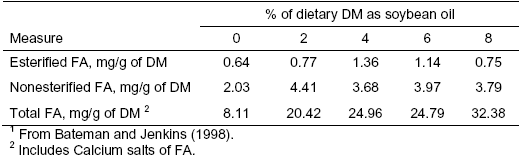
Total RUFAL could be divided into the two following ruminal pools: esterified and nonesterified. The nonesterfied fatty acid component of RUFAL likely has a more detrimental effect on the microbial population and MFD than the esterified component of RUFAL. Unfortunately, very little published data is available on distribution of fatty acids in the rumen between the esterified and nonesterified pools. In one example (Table 2), the concentration of nonesterified fatty acids in ruminal contents increased from 2.03 to as high as 4.41 mg/g DM as soybean oil added to the diet increased from 0 to 8 per cent (Bateman and Jenkins, 1998).
Low Rumen pH
Factors that can result in marked changes in ruminal pH through any 24-h period include: dietary carbohydrate profile and rates of degradation of the carbohydrate fractions as affected by source, processing, and moisture; physically effective NDF (peNDF) supply as affected by source and particle size; and production of salivary buffers as a function of peNDF supply and source (Shaver, 2005). Despite our general understanding of these factors, the degree and duration of low ruminal pH required to cause sufficient flux of PUFA through alternative pathways of ruminal BH is not known. Although data are limited, changes in rumen pH are most likely associated with MFD because they cause a change in the bacterial population favoring those that have alternative BH pathways.
Martin and Jenkins (2002) examined continuous culture incubations that were conducted at dilution rates of 0.05 and 0.10/h with pH values of 5.5 and 6.5, and 0.5 and 1.0 g/L of mixed soluble carbohydrate. They found that the most influencial environmental factor on formation of CLA and trans-C18:1 from linoleic acid was culture pH. At pH 5.5, the concentration of trans-C18:1 and CLA were significantly reduced. Similar effects were observed by Troegeler-Meynadier et al. (2003). They found that the amounts of BH products were always lower with pH 6.0 than with pH 7.0 in 24 h in vitro incubations. Low amounts of CLA at pH 6.0 could be due to low isomerase activity or to high reductase activity. Moreover, they found that low pH (pH 6.0) resulted in lower amount of trans-11 C18:1 at all incubation times compared with higher pH (pH 7.0), but concentration of trans-10 C18:1 was higher at 16 to 24 h of incubation. Low pH inhibited initial isomerization and the second reduction (trans-11 C18:1 to stearic acid), leading to an accumulation of trans-11 C18:1 in ruminal cultures (Troegeler-Meynadier et al., 2006). Choi et al. (2005) reported that cis-9 trans-11 CLA are produced at pHs higher than 6.2 by rumen bacteria, but trans-10 cis-12 CLA are produced more than cis-9 trans-11 CLA at lower pH. They concluded that trans-10 cis-12 CLA producing bacteria may be more aero and acid-tolerant than cis-9 trans-11 CLA producing bacteria.
Oleic acid BH was also affected by ruminal pH and dilution rate. AbuGhazaleh et al. (2005) reported that low pH and dilution rate restricted formation of trans-C18:1 and increased the concentration of stearic acid from oleic acid. They conducted the experiment using 13C-labeled oleic acid to determine BH intermediates in mixed ruminal microorganisms grown in continuous cultures at different pH and liquid dilution rate. At pH 6.5 and 0.10/h dilution rate, 13C enrichment was detected in trans-6 through trans-16 C18:1 isomers. However, at pH 5.5 and 0.05/h dilution rate, 13C enrichment was not detected in any trans isomers with a double bond position over C10.
Qiu et al. (2004) reported that reduced ruminal pH can affect microbial populations, especially cellulolytic bacteria. Total cellulolytic bacteria numbers are reduced, accompanied by reduced acetate-to-propionate ratio and altered BH when pH was low. The rumen pH also influenced fungal growth and metabolism. Culturing ruminal fungi at pH 6.0 and pH 7.0 slowed BH compared with pH 6.5. CLA production was increased by pH 7.0 compared to pH 6.0 and pH 6.5. Therefore, optimum pH was 6.5 and 7.0 for BH and CLA production, respectively, by ruminal fungi (Nam and Garnsworthy, 2007).
Monensin
Ionophores disrupt ruminal BH similar to unsaturated fat supplements. Higher concentrations of linoleic acid, trans C18:1, and CLA were maintained in continuous cultures of ruminal bacteria following infusion of monensin, nigericin, or tetronasin (Fellner et al., 1997). Feeding monensin had similar effects as fat supplements on enhancing linoleic acid and trans FA in milk of lactating cows, and also caused a reduction in milk fat percentage (Sauer et al., 1998).
Because of the antimicrobial nature of monensin and other ionophores, it is reasonable to expect that they function according to the BH theory and cause a microbial shift in the rumen that alters the pathways of BH. Monensin increases maintenance requirements of gram positive bacteria in the rumen which renders these bacteria less competitive in the ruminal environment (Duffield and Bagg, 2000). Ruminal microorganisms capable of fatty acid hydrogenation are often divided into Groups A and B based on their end-products and patterns of isomerization during BH (Harfoot and Hazlewood, 1997). Bacterial species in Group A hydrogenate linoleic acid to trans C18:1 but appear incapable of hydrogenating monoenes. Group B bacteria can hydrogenate a wide range of monoenes, including trans-11 C18:1, to stearic acid. The few bacterial species in Group B are gram-positive. Thus decreasing the number of bacteria that can carry out this process can potentially lead to a ‘build-up’ of BH intermediates in the rumen. This was highlighted by Fellner et al. (1997) when they examined the effect of monensin following continuous infusion of linoleic acid into rumen fermentors. With an unsupplemented diet the rate of 18:0 formation was 7.5 mg/L/hr whereas this decreased to only 2.7 mg/L/hr when monensin was supplemented (Fellner et al., 1997). It is important to remember, however, that an increased rumen outflow of BH intermediates will not be a problem if typical BH pathways are present. However, even if a small proportion of dietary PUFA are being hydrogenated through pathways that produce trans-10, cis-12 CLA and related intermediates, monensin can potentially increase the risk of MFD.
A recent on-farm epidemiological study was done in 2008 (Nydam et al., 2008) to establish risk factors that contribute to MFD in commercial dairy herds feeding Rumensin. This was an extensive study involving 79 commercial dairy herds across 10 states. Cow numbers ranged from 30 to 2800 across herds, with a mean herd size of 474 cows. Milk fat percentage ranged from 2.7 to 4.3 (mean 3.43 per cent) and Rumensin dose ranged from 150 to 410 mg/head/day (mean 258 mg/head/day). No significant associations with herd milk fat per cent were seen with stall types, cooling systems, or feeding design. Herds with higher formulated DMI, however, tended to have lower milk fat per cent. No relationships were seen between Rumensin dose and herd milk fat per cent. Likewise TMR concentrations of DM, ADF, NDF, NFC, and crude fat did not relate to milk fat per cent.
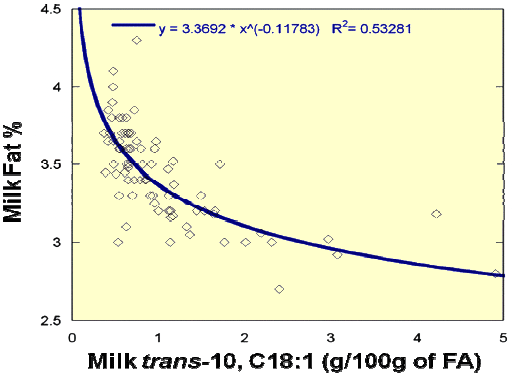
Several significant relationships did surface from the study. Herd milk fat per cent was strongly associated with changes in trans-10 C18:1 isomers in milk fat (Figure 2). An identical relationship was reported by Hinrichsen et al. (2006) between milk fat yield and milk trans-10 C18:1 across published research studies.
A significant relationship was also seen between TMR particle size and milk fat per cent. Separation of TMR samples into three particle size ranges using the Penn State Particle Separator (PSPS; 3-box, 2-sieve) gave significant relationships between milk fat per cent and particle size that were positive for the middle screen (P =0.008) and negative for the bottom pan (P=0.003). Milk fat per cent did not relate to TMR particle size on the top screen. No single TMR characteristic or ration component measured in this study accounted for more than 10 per cent of the variation in herd milk fat per cent. When a multi-variate regression was done on all measured TMR variables (e.g. TMR variables such as DM, starch, NDF, etc) and herd management factors, followed by a step-wise regression that eliminated non-significant variables one at a time, the significant factors remaining in the model were TMR DM per cent and percentage particles on the bottom pan of the PSPS. Together TMR DM per cent and bottom pan particles accounted for 21 per cent of the variation in herd milk fat per cent in the study.
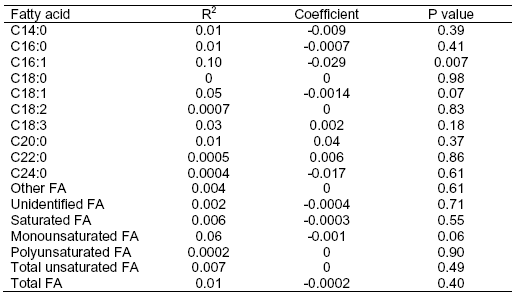
All individual fatty acids in the TMR were examined for possible relationship with milk fat per cent (Table 3). Unexpectedly, polyunsaturated fatty acid intake, including linoleic and linolenic acids, did not relate to milk fat per cent. It was expected that increasing intake of polyunsaturated fatty acids would cause a microbial shift and alter the pathways of BH leading to increased accumulation of CLA in the rumen that are associated with MFD. Rather surprisingly, only intakes of the monounsaturated fatty acids, C16:1 and C18:1, related to milk fat per cent.
Further Reading
| - | You can view a list of the full IFAS 20th Symposium proceedings by clicking here. |
February 2009


